Cooling Curve of Cetyl alcohol
An experiment was carried out where cetyl alcohol and water were allowed to cool from around 75 °C. The temperature of both substances was monitored every 30 seconds for 20 minutes. The results were plotted on a graph, which is given below.
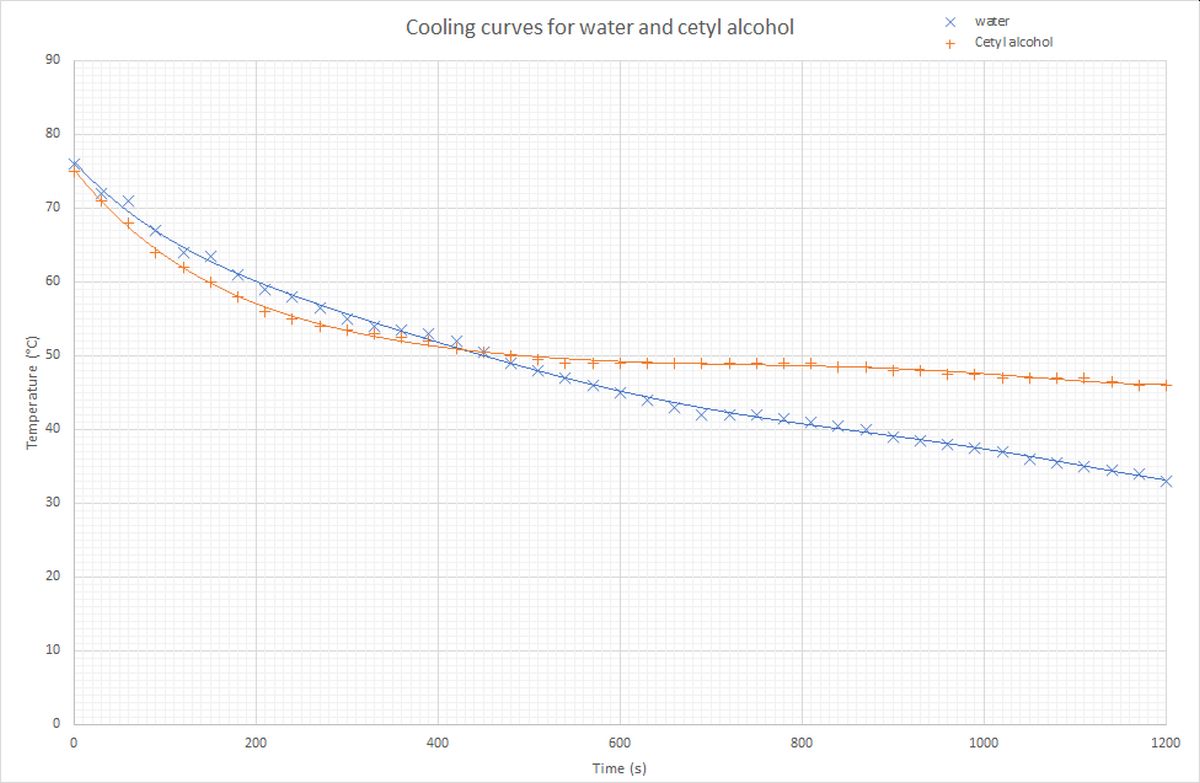
It can bee seen in the graph that the water continues to cool for the whole 20 minutes. The cetyl alcohol stays at 49 °C for over 5 minutes. The solid alcohol was seen to form at during this time. This means that the latent heat of fusion was being given out at 49 °C, which must be the melting point of cetyl alcohol.
Mandatory Knowledge
Knowledge that different materials require different quantities of heat to raise the temperature of unit mass by one degree Celsius.
Use of an appropriate relationship to solve problems involving mass, heat energy, temperature change and specific heat capacity.
Knowledge that the temperature of a substance is a measure of the mean kinetic energy of its particles.
Use of the principle of conservation of energy to determine heat transfer.
Knowledge that different materials require different quantities of heat to change the state of unit mass.
Knowledge that the same material requires different quantities of heat to change the state of unit mass from solid to liquid (fusion) and to change the state of unit mass from liquid to gas (vaporisation).
Use of an appropriate relationship to solve problems involving mass, heat energy and specific latent heat.
Last updated: 27/04/2023
